2020 WAEC CHEMISTRY PRACTICAL QUESTIONS AND ANSWERS AVAILABLE NOW
2020 COMPLETE CHEMISTRY QUESTIONS & ANSWERS NOW AVAILABLE. subscribe for your chemistry expo now if you don’t want late answers
==================================
*IMPORTANT INFORMATION:*
Remember to use your school’s average titre value for no 1.
[img]https://i.imgur.com/M1DZUPS.jpg[/img]
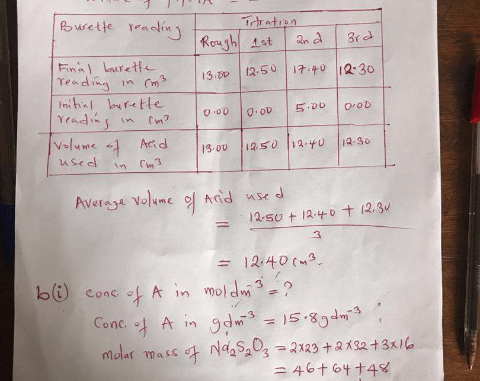
(1a)
In a tabular form
Burette Reading|1st reading|2nd reading|3rd reading
Final |15.25|0.00|45.79|
Initial|0.00||15.25|30.53|
Volume of acid used |15.25|15.28|15.26
Average volume of acid used = 15.25 + 15.26/2
= 15.255cm³
=15.26cm³
(1bi)
Given: Mass of conc of A = 5g/500cm³ = 5g/0.5dm³
Ca = 10g/dm³
A is HNO3
Therefore; Molar mass = 1+14+(16×3)
= 15+48
=63g/mol
Molarity of A = gram conc/molar mass
Ca = 10/63 = 0.1587mol/dm³
(1bii)
Using CaVa/CbVb = nA/nB
With reacting equation:
HNO3 +NaOH–>NaNO3 + H2O
nA = 1, nB = 1
0.1587×15.26/Cb×25.00 = 1/1
25Cb = 0.1587×15.26
CB = 0.1587×15.26/25
CB = 0.09687mol/dm³
(1biii)
B is NaOH
Molar mass = 23+16+1
=40g/mol
Conc of B in g/dm³ = molarity×molar mass
=0.09687×40
=3.8748g/dm³
(1biv)
No of moles present in 250cm³ of NaOH is
= molar conc. × volume
= 0.09687 × 250/1000
= 0.0242 moles
Mole ratio of NaOH and NaNO3 is 1:1
No of moles of NaNO3 which reacted is 0.0242
Mass of NaNO3 formed = molar mass × no of moles
= 85 × 0.0242
=2.057grams
===========================
[img]https://i.ibb.co/C7cHvy4/IMG-20200818-WA0020.jpg[/img]
(2a)
TEST
C + burning splint
OBSERVATION
Sample C bursts into flame
It burns with non-smoking blue flame, without soot.
Colourless gas that turns wet with blue litmus paper faint red and turns lime water milky is present
INFERENCE
C is volatile and flammable. The gas is CO2 from combustion of a saturated organic compound.
(2bi)
TEST
C + distilled water + Shake
OBSERVATION
Clear or colourless solution is observed
INFERENCE
C is miscible with water
(2bii)
TEST
C + Acidified K2Cr2O7
OBSERVATION
Orange color of K2Cr2O7 Solution turns pale green and eventually pale blue on cooling
INFERENCE
C is a reducing agent
(2c)
TEST
D + C + 10% NaOH + Shake
OBSERVATION
D dissolve slowly in C and produces reddish brown solution
Reddish brown solution turns yellow precipitate. The precipitate has an antiseptic odour
INFERENCE
D is soluble in organic solvents
Ethanol, ethanal or a secondary alkanol is present
(2d)
Compound belongs to the class of secondary alkanol
=================================
(3ai)
Zinc nitrate
(3aii)
2 Zn(NO 3 )2 —–>2 ZnO + 4 NO 2 + O 2
(3aiii)
It turns white when cold from it yellow colour when it was hot
(3b)
Pipette / measure 50.0cm3 of the stock solution into a 250
cm3 volumetric flask (containing some distilled water). Shake / swirl and add more distilled water until the mark is reached.
(3c)
Al2(SO4)3 – turns blue litmus red
=================================
Completed!!!!!!!
+++++++++++++++++++++++++++++++++
Answers Loading…………
+++++++++++++++++++++++++++++++++
(3ai)
Zinctrioxonitrate (v) – Zn(NO3)2
(3aii)
2Zn(NO3)2(g) –> 2ZnO(s) + 4NO2(g) + O2(g)
(3aiii)
The residue when it has yellow colour which will turn white on cooling
(3b)
Given; M1 = 1.0mol/dm³
V1 = ?
M2 = 0.2mol/dm³
V2 = 250cm³
Using M1V1 = M2V2
1 × V1 = 0.2×250
V1 = 50cm³
Procedure: Measure out 50cm³ of the stock solution, dilute it to 0.2mol/dm³ by adding 200cm³ of water.
(3c)
Al(SO4)3 will turn blue litmus paper red
=================================
Completed!!!!!!!
READ BELOW MESSAGES TO LEARN HOW TO SUBSCRIBE .
WHATSAPP/CALL 08160277754 FOR MORE INFO
==================================================
CLICK HERE TO VIEW THE ANSWERS
==================================================
ADVICE: Always subscribe at night before your exam date, so that we can have time to record your details before the exam so that your details will record on time. ANSWERS WILL BE SENT TO OUR SUBSCRIBERS 3hrs BEFORE THE EXAM
EXPOLORD.ORG TEAM ASSURE YOU NOTHING LESS THAN A1 IN THIS SUBJECT..ALL YOU NEED TO DO IS TO SUBSCRIBE.
Read below carefully to know how to subscribe for it.
-=-=-=-=-=-=-=HOW TO SUBSCRIBE=-=-=-=-=-=-=
CHEMISTRY EXPO PAYMENT
DIRECT TO MOBILE (SMS): 800 Mtn card
On this Direct mobile package we will send the Obj & Essay directly to your submitted phone numbers as early as we can…
Send 800 MTN CARD to 08160277754 + Subject name + phone number + i paid for direct sms
WHATSAPP EXPO: 500Mtn card
On this WHATSAPP EXPO package we will send the Obj & Essay directly to the whatsapp phone number you submitted to us 3hrs before the exam time.
Send 700 MTN CARD to 08160277754 + Subject name + phone number + i paid for direct sms
PASSWORD LINK: 400mtn card
On this package of subscription, We will send a Password directly to your phone number, which will be Used to access the Answers Online. on our ANSWER PORTAL.
Send N400 MTN CARD to 08160277754 + Subject name + phone number with message i paid for online password
Our Online/ Answers page Link www.A1answers.com
Our Subscription Price List For Direct Mobile.
(i) Maths & English: N1000 each
(ii) Per Subject: N800 each
Our Subscription Price List for WhatsApp Message.
(i) Per Subject: N600 each
(ii) Maths & English: N7000 each
Our Subscription Price List For Password Answers.
(i) Per Subject: N400 each
(ii) practicals Per Subject: N400 each
(iii) Maths & English: N500 each
HOW TO MAKE PAYMENT
Send
(i) Payment name/Your name.
(ii) Subject Name (e.g CHEMISTRY)
(iii) MTN Recharge Card Pin(s)
(iv) Phone number to 08160277754
Remember Success is not a must, is an Option.
Don’t let this little amount of money make you fail this exam.
WARNING:-Please Don’t Even Come for Free Answers, because it Would’nt be Posted. Take me Serious This Time.
Make Sure you Subscribe if you Don’t Want to be on Hot Seat

Leave a Reply